
The ‘rapture of the deep’, the martini-effect, getting ‘narced’ – gas narcosis goes by many names. But what is it that causes narcosis? Can we adapt to it? Is it caused by gases other than nitrogen? In this installment of our Education series, we dive deep into narcosis, its causes, and methods of prevention.
Narcosis: What’s in a Name?

It’s likely that you first learnt of narcosis as ‘nitrogen narcosis’. This is because most divers only ever dive with air or nitrox and as nitrogen is largely responsible for the narcotic effect, it’s become part of the name.
However, nitrogen is not the only gas that can cause narcosis. In fact, all breathable gases have narcotic potency, except for helium and neon. Therefore, it is more accurate to say ‘gas narcosis’.
Symptoms

Gas narcosis is effectively the breathing of an anesthetic gas at a subanesthetic dose. In other words, to be narced is to be under the effect of an anesthetic gas, just the same as when you need dental or bodily surgery. Obviously, this can pose a risk when underwater, as the last thing we want to be when diving is unconscious or near to it.
Narcosis has been compared to alcohol, hence its nickname of ‘the martini-effect’. Essentially, for every ten metres deeper you go, it’s said to be the equivalent of drinking another martini. This is an effective analogy to begin with, but, as you will see, narcosis is a little more complex than simply being drunk.
There are multiple symptoms of narcosis and they can often vary from person to person. However, we can broadly categorise symptoms into three subdivisions.
- Impaired mental function
- Impaired neuromuscular coordination
- Subjective experiences
The most common symptoms reported by divers are:
- Delayed response to visual stimuli
- Euphoria
- Tunnel vision (both literal and metaphorical)
- Impaired reasoning, judgement and decision making
- Over-confidence
- Anxiety & paranoia
- Sleepiness
In more extreme and less common situations, divers have also reported:
- Hallucinations
- Fear
- Dizziness and vertigo
- Severe depression
- Severe panic or manic states
- Unconsciousness
Clearly, all of these things increase our risk of having an accident underwater. The Narcotic Mechanism
While the exact mechanism of narcosis is not completely understood, there is a general theory of why and how it occurs.
In the central nervous system, there are cells called ‘neurons’ which send information to other nerve and muscle cells. In order to transmit this information, neurons send an electrical impulse between the contact points of the cells. These contact points are called ‘synapses’. Synapses carry these messages between neurons which are called ‘action potentials’.
Anesthetic gases disrupt the synaptic transmission of these action potentials. They do this by stimulating the activity of inhibitory neurotransmitters, which reduce the frequency of action potentials. Therefore, the central nervous system is depressed, resulting in an anesthetic effect.
Before this theory, the explanation of narcosis was based on the Meyer-Overton Rule. This stated that narcotic potency was based on the lipid solubility of the gas being breathed. Solubility refers to the ability of a substance to be dissolved. It was believed that narcotic gas was absorbed by the fatty sheath (called the Myelin sheath) surrounding the nerve cell, which in turn inhibited the transmission of action potentials. Although this theory has now changed, the Meyer-Overton rule is still correct insofar as we know narcosis disrupts the transmission of action potentials.
Can We Adapt to it?
It is often said by more experienced divers that beginners have to build a ‘tolerance’ to nitrogen in order to better deal with narcosis. But is this actually possible?
There are divers who can dive to 50 metres and be relatively clear-headed. There are also divers who start to feel narced even before they reach 30 metres. However, this is the result of individual biological predisposition – not a difference in experience or an accumulated tolerance. Similarly, susceptibility to narcosis can differ within an individual due to environmental factors such as drug intake, energy levels, water conditions, and many other factors.
Drug tolerance occurs when repeated exposure to a drug results in a decrease in its effects on the user. For example, someone who is used to drinking alcohol tends to require more to feel its effect than someone trying it for the first time.
Experimental evidence suggests that repeated exposure to the narcotic effects of gases does not reduce the anesthetic potency of the gas. Therefore, it is unlikely that we are able to develop an actual tolerance to narcosis, in the same sense as that of a drug tolerance. We will be just as narced on our first exposure as on our five-hundredth.
However, what does seem to change with repeated exposure is the subjective perception of the diver. While they are just as cognitively impaired and neuromuscularly uncoordinated as on their first exposure, they become more aware that they are indeed under the influence of narcosis.
This would suggest that repeated exposure results in an adaptation, rather than a tolerance. An adaptation is a change in behaviour that results in an increase in performance in a given environment. Thus, although the impairment level remains the same, the diver’s subjective perception of the narcotic effects will change.
Predicting Narcosis

Narcosis is dependent on three factors:
- The gas being breathed (e.g. air, nitrox, trimix)
- The percentage of the narcotic gas present in the overall gas mixture (e.g. 79% nitrogen in air)
- The pressure of the gas (i.e. the depth)
These are all closely related to one another and it is worth taking some time to look at each of them individually. Firstly, let’s look at the gas being breathed.
Choice of Gas
Different gases have different ‘narcotic potencies’. The narcotic potency of a gas is simply the strength of its narcotic effect in a given dose. It can be thought of in the same way the percentage of alcohol determines the strength of a drink.
For example, beer tends to have a lower percentage of alcohol present, usually around 5% of the total volume, whereas vodka usually has at least 40% or higher. Therefore, vodka has a higher ‘narcotic potency’ than beer. This is exactly the same with breathing gases. The table below shows the relative narcotic potency of various gases:
| Gas | Relative Narcotic Potency |
| Helium | 0.045 |
| Neon | 0.3 |
| Hydrogen | 0.6 |
| Nitrogen | 1.0 |
| Oxygen | 1.7 |
| Carbon Dioxide | 20.0 |
As it can be seen, helium has a very low narcotic potency while carbon dioxide has a very high one. Also, perhaps surprisingly, you can see that oxygen has a higher narcotic potency than nitrogen – we’ll come back to this later.
For now, we can see clearly how narcotic potency is a factor to consider in gas choice.
Fraction of Narcotic Gas
The fraction of narcotic gas in a gas mixture simply refers to the percentage narcotic gas accounts for in the total mixture.
As we’ve already established, different gases have different narcotic potencies. Thus, the greater the fraction of narcotic gas in a mixture is, the greater the narcosis will be.
It is for this reason that technical divers begin to add helium to their gas mixtures the deeper they go, in order to reduce the fraction of nitrogen. For example, a common trimix blend (helium/oxygen/nitrogen) is Trimix 20/40. The fractions of gas in this mixture are:
- 40% Helium
- 40 % Nitrogen
- 20% Oxygen
While air has 79% nitrogen, Trimix 20/40 has only 40%. Clearly, compared to diving with normal air, diving with Trimix 20/40 will significantly reduce narcosis.
Partial Pressure of Narcotic Gas
The higher the partial pressure of the narcotic gas, the greater the level of narcosis.
The partial pressures of nitrogen and oxygen in air, at surface pressure, are:
- 0.21 Oxygen
- 0.79 Nitrogen
To calculate the partial pressure of a gas at any given depth, we simply multiply surface pressure value by the depth expressed in pressure (bar). So the partial pressures of nitrogen and oxygen at 30 metres (4 bar), for example, are:
- Oxygen = 0.21 x 4 = 0.84 bar
- Nitrogen = 0.79 x 4 = 3.16 bar
Most training agencies put limitations on the maximum depth you can dive to on air. This is usually between 40-55 metres. From this, you can then calculate what the partial pressure of narcotic gases will be at that depth. This will give you the maximum allowable narcotic gas partial pressure in any gas mix, also known as the ‘Equivalent Narcotic Depth’ (END).
For example, if the air diving limit is set at 40 metres, the partial pressures are:
- Oxygen = 0.21 x 5 = 1.05 bar
- Nitrogen = 0.79 x 5 = 3.95 bar
From here, if we take oxygen to also be narcotic, then the maximum allowable narcotic gas partial pressure is the sum of the two partial pressures:
1.05 + 3.95 = 5 bar
Thus, in this example, the maximum allowable narcotic gas partial pressure in any gas mix (or END) would be capped at 5 bar.
Why is this useful? You may ask.
If we are diving beyond 40 metres, past the air limit, we have to use trimix in order to reduce the nitrogen content and stay below the maximum narcotic gas partial pressure. Therefore, when calculating how much helium to add to our gas mixture, we now know that the combined partial pressures of oxygen and nitrogen (the narcotic gases) need to be at least equal to or below 5 bar.
For example, if we dive to 60 metres (7 bar) using Trimix 20/40, the partial pressures of the gases at that depth will be:
- Helium = 0.4 x 7 = 2.8 bar
- Nitrogen = 0.4 x 7 = 2.8 bar
- Oxygen = 0.2 x 7 = 1.4 bar
This means the narcotic partial pressure will be:
2.8 + 1.4 = 4.2 bar
Therefore, we know that we can safely dive to 60 metres with this gas, as we are below the maximum narcotic partial pressure limit of 5 bar.
On the other hand, if we were to dive to 60 metres using Trimix 20/20, the partial pressures of the gases at that depth will be:
- Helium = 0.2 x 7 = 1.4 bar
- Nitrogen = 0.6 x 7 = 4.2 bar
- Oxygen = 0.2 x 7 = 1.4 bar
In this case, the narcotic partial pressure will be:
4.2 + 1.4 = 5.6 bar
Clearly, Trimix 20/20 would be unsuitable to dive to 60 metres with, in this example, as our equivalent narcotic depth (END) would be 46 metres.
As mentioned, this value is called the ‘Equivalent Narcotic Depth’ (END). Simply, this means ‘when I breathe x gas at y depth it is the equivalent of breathing air at z depth.’ Using our example above, breathing Trimix 20/40 at 60 metres is the equivalent of breathing air at 32 metres, because the combined narcotic partial pressure is 4.2 bar (the ambient pressure at 32 metres). Equally, breathing Trimix 20/20 at 60 metres is the equivalent of breathing air at 46 metres.
Oxygen

As previously mentioned, oxygen actually has a narcotic potency almost double that of nitrogen, based on its lipid solubility. Yet, why is this not talked about as much? Why is it not named ‘oxygen narcosis’ instead?
While oxygen may have a higher narcotic potency than nitrogen, it is never breathed at a partial pressure as high as that of nitrogen. Oxygen is capped to a maximum partial pressure of 1.6 bar. Often, however, oxygen partial pressures will be much lower.
For example, on an air dive at 40 metres (5 bar), the partial pressures of the gases you are breathing are:
- Nitrogen = 0.79 x 5 = 3.95 bar
- Oxygen = 0.21 x 5 – 1.05 bar
On top of this, the extent of the narcotic potency of oxygen is still largely unknown as research and experiments are difficult to conduct. In order to test oxygen’s narcotic potency, divers would have to be exposed to very high partial pressures of oxygen, well beyond the limit of what is safe regarding oxygen toxicity. This would mean chamber dives (simulated dry ‘dives’ inside a hyperbaric chamber) would have to be used instead, to eliminate the risk of drowning. However, it is well-documented that the human body reacts very differently to oxygen in a dry environment compared to when submerged in a wet environment. Therefore, the scope of these experiments may be limited.
This being said, it’s still a conservative measure to include oxygen in narcosis calculations. At best, it will reduce narcosis and thereby increase safety. At worst, it will increase the cost of your gas if using helium.
The agencies who include oxygen in their narcotic calculations are Global Underwater Explorers (GUE), PADI, CMAS and PSAI. Agencies who don’t include oxygen as a narcotic gas are IANTD, TDI, ANDI, NAUI and BSAC. SSI takes the mid stance that oxygen is narcotic, but far less than nitrogen in the dosages we are exposed to.
Carbon Dioxide
Carbon dioxide is the end-product of respiration and is thus natural to our bodies. However, an excessive build-up of carbon dioxide (hypercapnia) can result in severe CO2 narcosis.
The symptoms of CO2 narcosis are:
- Dizziness
- Nausea
- Headaches
- Shortness of breath
- Hyperventilation
- Loss of consciousness
There are two primary causes of CO2 narcosis:
- Exertion
- Breathing a dense gas
To understand how these two factors cause narcosis, we need to understand the process of breathing.
The Process of Breathing and Carbon Dioxide Build-Up
Our rate of ventilation is controlled by the partial pressure of CO2 (PCO2) in our blood. Our central nervous system adjusts our rate of breathing in order to maintain a constant PCO2.
With increased exertion, we will metabolise oxygen at a higher rate, leading to an increase in CO2 being produced. Thus, our ventilation rate will increase in order to remove the excess CO2 produced, thereby maintaining a constant PCO2. When our exertion rate decreases, leading to a lower rate of metabolization, the reverse occurs. It is a homeostatic process.
However, when we are exposed to a hyperbaric environment, this process is hindered.
Inhalation and exhalation are governed by Boyle’s Law (pressure and volume are inversely proportional). In order to inhale, our diaphragm and intercostal muscles contract resulting in an increase in total lung volume. This increase in volume causes a decrease in pressure in the thoracic cavity, causing it to have a lower pressure than the atmospheric pressure. Thus a pressure gradient exists between the atmosphere and thoracic cavity, resulting in an inflow of air into the lungs: inhalation. The reverse applies for exhalation: the diaphragm relaxes, reducing volume, thereby increasing the pressure of the thoracic cavity relative to the atmosphere, resulting in an outward flow of air.
However, there exists a maximum limit to how quickly air can be vented from the lungs: the maximum expiratory gas flow rate (MEGFR). This is because during high level exertion, we begin to forcefully exhale, thereby increasing intrathoracic pressure on the outside of our airways. There comes a point at which the intrathoracic pressure becomes so high that it results in the collapse of the airways, thereby slowing exhalation. Thus, exhalation is termed ‘effort independent’ as we cannot overcome this and exhale more and more air purely by creating more effort. The diagram below may help you to visualise this.
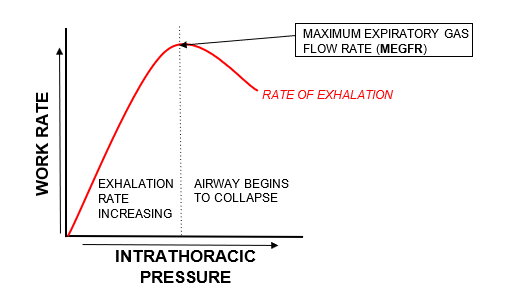
The amount of work required to exhale gas is fixed and equal under both normobaric and hyperbaric conditions. In other words, it is effort independent. We cannot overcome the MEGFR and exhale more gas purely by creating more ‘work’. Therefore, when we breathe a denser gas (>1 bar), we are unable to increase the work rate of exhalation, meaning that we are using the same work rate to now move a heavier substance. Thus, the MEGFR will be reduced. The diagram below demonstrates this inverse relationship.
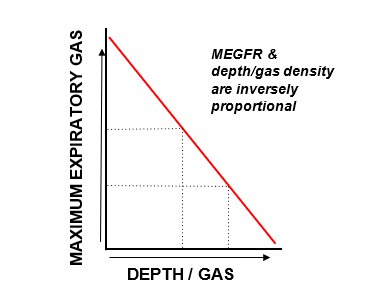
So, what does all this mean for us as divers?
If we are diving with minimal exertion at a depth of say 30m (4 ATA), then our respiratory system will be able to ventilate and maintain a constant PCO2 as if we were at the surface (1 ATA), despite breathing a gas four times as dense. However, if we were to then engage in significant physical exertion at this depth, our respiratory system is effectively capped by the maximum ventilation work rate sustained at surface pressure (1 bar), meaning that excess CO2 would not be able to be cleared at a quick enough rate to maintain a constant PCO2. Therefore, bodily PCO2 will rise, leading to CO2 narcosis. This then leads to a vicious cycle, as increased PCO2 leads to increased work of breathing, which in turn increases PCO2 levels further – worsening the narcotic effect.
What makes CO2 narcosis particularly dangerous, compared to that of oxygen and nitrogen, is that it can happen at any depth. All that is required for it to occur is exertion coupled with the breathing of a gas denser than that at surface pressure (which happens as soon as you’re deeper than 0 metres).
The procedure for dealing with CO2 narcosis is firstly to reduce exertion and calm down. If this is not possible, or does not improve the situation, then ascending to a shallower depth will aid by reducing the density of the gas breathed.
Overall, gas narcosis poses a real danger to those of us diving at the deeper end of the spectrum. Not only can it be caused by nitrogen, but also by oxygen and carbon dioxide too. Similarly, unlike the other gases, carbon dioxide narcosis is depth independent. For the conservative, thinking diver, narcosis is a key factor to consider when planning and executing a dive.